You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
460 nm vs 420 nm
- Thread starter WRASSER
- Start date
460 is more blue and less violet than 420. The reason why 420 is so popular (the standardized actinic wavelength) is because this was the wavelength proven to stimulate the most photosynthesis in Chlorophyll A. Corals and plants thus likely derive the most energy from this spectrum than any other. I'm sure 460 is used effectively too.
Another thing is they'll fluoresce different pigments, so some greens will look more neon green, some pinks more pink, etc.
Another thing is they'll fluoresce different pigments, so some greens will look more neon green, some pinks more pink, etc.
Len,
Do you think this bulb is what we would call actinic?

Snippet from internet, posted by a Japanese reef keeper.
Might be one to supplement mini MH pendants...
Do you think this bulb is what we would call actinic?

Snippet from internet, posted by a Japanese reef keeper.
BB450 is fluorescent light which is 420nm-470nm peak enhanced amd its light looks deep blue.
Might be one to supplement mini MH pendants...
I don't know as there isn't a set-in-stone definition for actinic wavelength, but probably not. Actinic refers to violet/near-UV light, which 420nm is on the cusp of. A few nm higher and you move into the blue area, so if the peak of that bulb is around 450nm, it's really just a blue bulb.
While both 460 & 420 will fluoresce, I'm a big fan of the 420 when it's alone simply because our eyes register the purple as really dark, as a result the glowing from the corals tends to pop a bit more due to the contrast between light and dark. 460 however typically is made a bit more efficiently in that the PAR is almost always going to be more on a bulb vs bulb comparison (i.e 54w 420nm t5 vs 54w 460nm t5) now whether or not that's better for the corals I think is still hotly debated.
My own thinking on corals has been coming closer to that of plants--we must mimic the sun.sfsuphysics":3c6qecxh said:While both 460 & 420 will fluoresce, I'm a big fan of the 420 when it's alone simply because our eyes register the purple as really dark, as a result the glowing from the corals tends to pop a bit more due to the contrast between light and dark. 460 however typically is made a bit more efficiently in that the PAR is almost always going to be more on a bulb vs bulb comparison (i.e 54w 420nm t5 vs 54w 460nm t5) now whether or not that's better for the corals I think is still hotly debated.
Thanks for that link, Len, I should have known there was a Wiki (no Wiki back in the 80s).
as usual you are the man Len, Your info is helpful once again. I am trading the 150 for a 220 and found some metal hilide with attinic. I am hearing that i I can run the the MH for 6 hours. Is that enough, i know it depends on what i am wanting to put in the tank. In general will that be long enough or too long?
seamaiden":wf2m7lec said:My own thinking on corals has been coming closer to that of plants--we must mimic the sun.
The problem(s) with that, the sun has a huge wide range of light it emits, spiking in the yellow/green region. Not to mention the color spectrum that plants use can differ than corals, hell within the plant kingdom there are species that prefer different ranges. Also corals have a barrier of ocean which changes the spectral properties of the light that gets to them. Not saying the Sun is a bad thing, but there is a reason why many reefers have pushed to the bluer end of the spectrum when it comes to lighting over their tanks, some of which is pretty too
True, but are you asserting that people light with a single wavelength? Every spectral graph of every MH bulb I've taken a look at shows peaks in other portions of the spectrum. This is what I'm getting at. Actinic lighting, if taken strictly, could mean just 420nm wavelength. It's like taking nothing but vitamin C and considering that a 'rounded diet'.
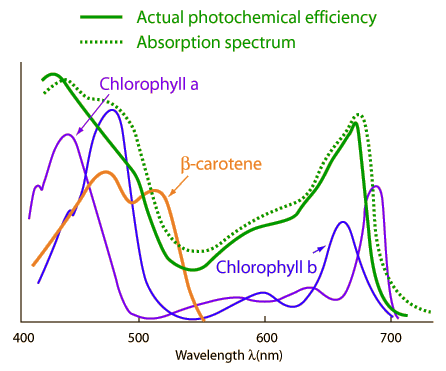
Different chlorophylls have different absorption spectra, and then there are loads of modifications that essentially enable plantlife to use pretty much everything in the visible spectrum.
Here is the absorption spectrum for Chlorophyll a:

It peaks at about 440 nm and drops to pretty much nothing by 460 nm
Here is the absorption spectrum for Chlorophyll a:

It peaks at about 440 nm and drops to pretty much nothing by 460 nm
Nice graphs there Subcom. They've inspired me to look something up in Delbeek and Sprung. This is from The Reef Aquarium Volume 1, P. 48):
The zooxanthellae of corals are brown in color, which is the best color for absorbing blue light (Bensosn, 1984). [...] The accessory pigments isolated fron zooxanthellae, such as cartenoids and several xanthophylls, all exhibit peak absorbtion between 408 and 475 nm.
Subcomandante Marcos":306s3rpf said:The sun exhibits near black-body radiation, which does not have a peak. It is a smooth, broad spectrum that has a maximum at about 5,500 Kelvin (the sun's surface temperature).
Your definition of peak must be a bit different than mine, because blackbody radiation just means it emits at all wavelengths, there is however a particular wavelength that does come out more than others however by a tad.
If I understand what Bill said, I think he kind of said that. Not a marked peak, but a gradual 'wave' is how the spectral graph appears to me. So, while it's not showing sharply defined peaks and valleys, it is still a sort of peak, yes? No? :?
I've been under the impression that these activities need more than just one or two specific wavelength peaks (or sufficient strength of a particular wavelength).sfsuphysics":35v434wq said:Well photosynthesis occurs at a particular wavelength or two doesn't it?
But yeah, lights typically have a sharp peak at a wavelength or two, however they often have additional light coming off them.
Yes, and this is why I find all artificial light to be lacking for my own current needs (which are not necessarily all that different from the aquatic application we're discussing here). Plasma bulbs, however, almost PERFECTLY mimic the sun. Damn that $1,500 price tag just for the bulb! <shakes fist> Then again, we do still have The Big Metal Halide in the Sky.Subcomandante Marcos":35v434wq said:The sun exhibits near black-body radiation, which does not have a peak. It is a smooth, broad spectrum that has a maximum at about 5,500 Kelvin (the sun's surface temperature).
seamaiden":79zmgidc said:I've been under the impression that these activities need more than just one or two specific wavelength peaks (or sufficient strength of a particular wavelength).
Yes, and this is why I find all artificial light to be lacking for my own current needs (which are not necessarily all that different from the aquatic application we're discussing here). Plasma bulbs, however, almost PERFECTLY mimic the sun. Damn that $1,500 price tag just for the bulb! <shakes fist> Then again, we do still have The Big Metal Halide in the Sky.Subcomandante Marcos":79zmgidc said:The sun exhibits near black-body radiation, which does not have a peak. It is a smooth, broad spectrum that has a maximum at about 5,500 Kelvin (the sun's surface temperature).
Have you looked at Ceramic Metal Halides?
Some of them offer a comparatively even spectrum:
Here is a graph comparing a Phipps MasterColor CMH to HPS http://www.growlightexpress.com/images/cmhvshps.jpg
What I don't like about sulfur plasma bulbs is the huge green spike.